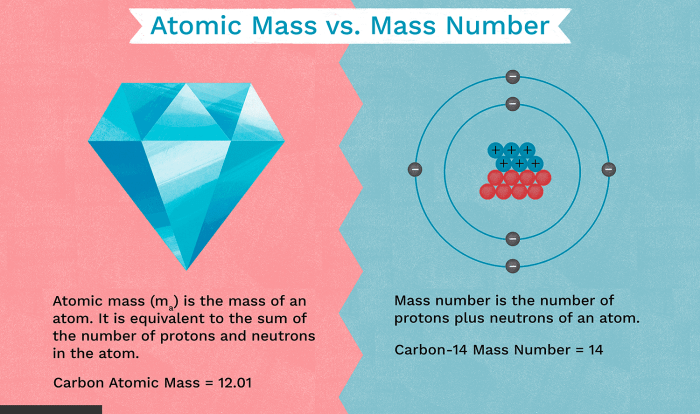
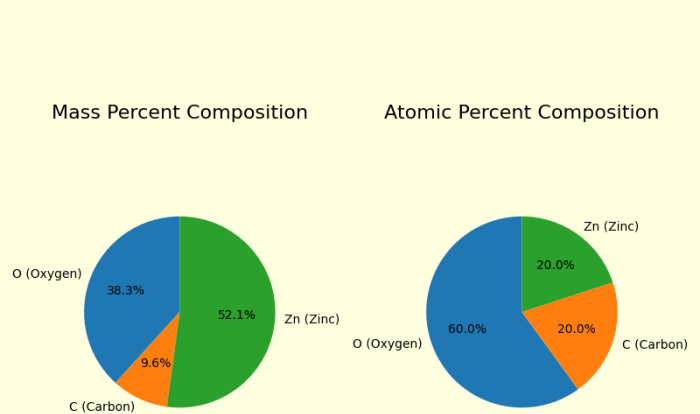
Welcome to the realm of gizmo balancing chemical equations answers, where we embark on a journey to unravel the intricacies of chemical reactions and master the art of balancing equations with precision. As we delve into this engaging exploration, we will uncover the secrets of stoichiometry, redox reactions, and advanced balancing techniques, empowering you with a comprehensive understanding of this fundamental aspect of chemistry.
Throughout this discourse, we will unravel the purpose and key concepts of the Gizmo simulation, providing a step-by-step guide to harness its capabilities. We will explore various methods for balancing chemical equations, including the trial-and-error and half-reaction methods, highlighting their advantages and drawbacks.
Real-world examples and applications will illuminate the significance of balanced equations in chemical reactions and their role in stoichiometric calculations.
1. Gizmo Balancing Chemical Equations
The Gizmo simulation provides a visual and interactive environment for students to learn about balancing chemical equations. It allows users to explore the concepts of stoichiometry and chemical reactions in a hands-on way.
Key concepts involved in balancing chemical equations include:
- Law of conservation of mass: Matter cannot be created or destroyed, so the number of atoms of each element must be the same on both sides of the equation.
- Coefficients: Coefficients are numbers placed in front of chemical formulas to balance the number of atoms of each element.
To use the Gizmo to balance equations, follow these steps:
- Enter the unbalanced equation into the Gizmo.
- Click on the “Balance” button.
- The Gizmo will automatically adjust the coefficients to balance the equation.
- Check the balanced equation to make sure that the number of atoms of each element is the same on both sides.
2. Methods for Balancing Chemical Equations
Trial-and-Error Method
The trial-and-error method is a simple and straightforward approach to balancing chemical equations. It involves repeatedly changing the coefficients until the equation is balanced. This method can be time-consuming, especially for complex equations.
Half-Reaction Method
The half-reaction method is a more systematic approach to balancing chemical equations. It involves breaking the equation into two half-reactions, one for oxidation and one for reduction. The half-reactions are then balanced separately, and the coefficients are adjusted to make the overall equation balanced.
Comparison of Methods, Gizmo balancing chemical equations answers
The trial-and-error method is easier to understand and use, but it can be time-consuming for complex equations. The half-reaction method is more systematic and efficient, but it can be more difficult to understand.
3. Examples and Applications
Real-World Examples
Balanced chemical equations are used in a wide variety of applications, including:
- Predicting the products of a chemical reaction
- Calculating the amount of reactants and products in a reaction
- Determining the limiting reactant in a reaction
Importance of Balancing Equations
Balancing chemical equations is important because it ensures that the law of conservation of mass is obeyed. This law states that matter cannot be created or destroyed, so the number of atoms of each element must be the same on both sides of the equation.
If an equation is not balanced, it is not a valid representation of the chemical reaction.
4. Advanced Concepts
Redox Reactions
Redox reactions are chemical reactions that involve the transfer of electrons. In order to balance redox reactions, it is necessary to use the concept of oxidation numbers.
Oxidation Numbers
Oxidation numbers are assigned to each atom in a molecule or ion to represent the number of electrons that it has gained or lost. Oxidation numbers can be used to determine which atoms are being oxidized and which are being reduced.
Complex Equations
Some chemical equations are very complex and require advanced balancing techniques. These techniques include the use of matrix algebra and computer programs.
5. Troubleshooting Tips
Common Errors
Some common errors made when balancing chemical equations include:
- Not checking the number of atoms of each element on both sides of the equation
- Using incorrect coefficients
- Forgetting to balance the charges on both sides of the equation
Debugging Strategies
If you are having trouble balancing an equation, try the following strategies:
- Start by balancing the elements that appear in the smallest number of molecules.
- Use the Gizmo to check your work.
- Seek help from a teacher or tutor.
Essential Questionnaire: Gizmo Balancing Chemical Equations Answers
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side of the equation equals the number of atoms of that element on the products’ side. This adherence to the law of conservation of mass is crucial for accurate stoichiometric calculations and understanding chemical reactions.
What are the different methods for balancing chemical equations?
Two common methods for balancing chemical equations are the trial-and-error method and the half-reaction method. The trial-and-error method involves adjusting the stoichiometric coefficients in front of each chemical formula until the equation is balanced. The half-reaction method involves breaking down the reaction into two half-reactions, balancing each half-reaction separately, and then combining them to obtain the overall balanced equation.
What is the importance of using the Gizmo simulation for balancing chemical equations?
The Gizmo simulation provides an interactive and visual representation of chemical reactions, allowing students to manipulate reactants and products to achieve a balanced equation. This hands-on approach enhances understanding and facilitates the learning process.