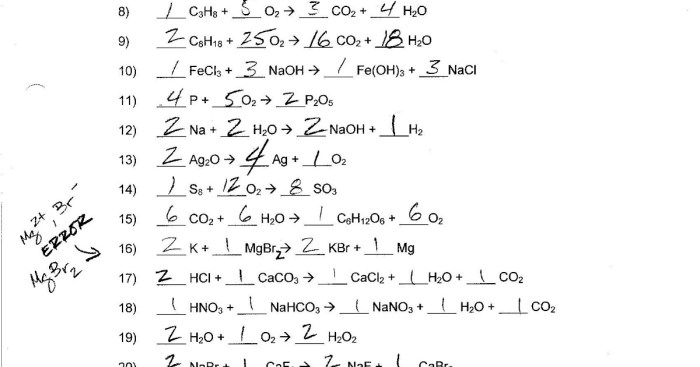Introducing the Atomic Number and Mass Number Worksheet, an invaluable resource designed to illuminate the intricacies of atomic structure. This worksheet delves into the concepts of atomic number and mass number, providing a comprehensive understanding of their significance in determining the identity and properties of elements.
Atomic number, a defining characteristic of each element, plays a pivotal role in distinguishing one element from another. Mass number, on the other hand, unveils the composition of an atom’s nucleus, offering insights into its isotopic variations. Together, these concepts form the cornerstone of our understanding of the atom and its behavior.
Atomic Number
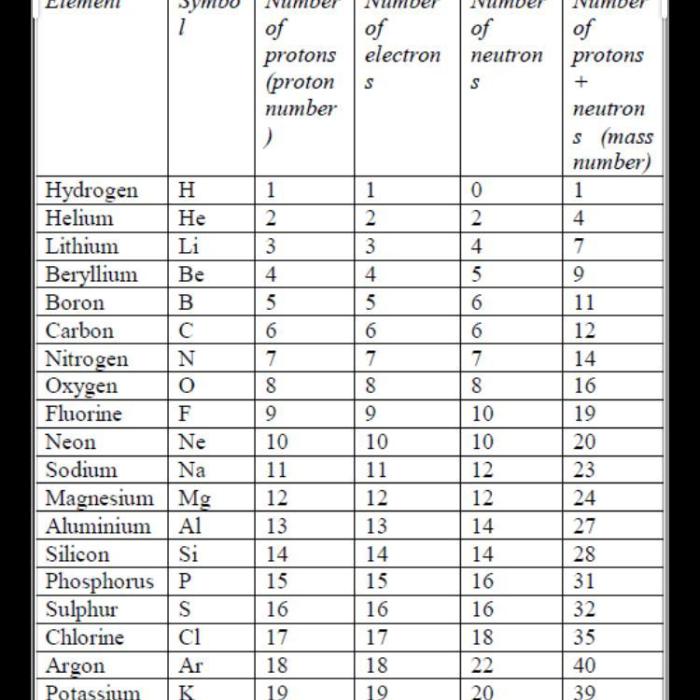
Atomic number is a fundamental property of an element that defines its identity and distinguishes it from all other elements. It represents the number of protons in the nucleus of an atom.
For example, the atomic number of hydrogen is 1, indicating that a hydrogen atom has one proton in its nucleus. The atomic number of oxygen is 8, indicating that an oxygen atom has eight protons in its nucleus.
The atomic number determines the element’s position on the periodic table and its chemical properties. Elements with the same atomic number belong to the same element, regardless of their mass number or the number of neutrons in the nucleus.
Mass Number: Atomic Number And Mass Number Worksheet
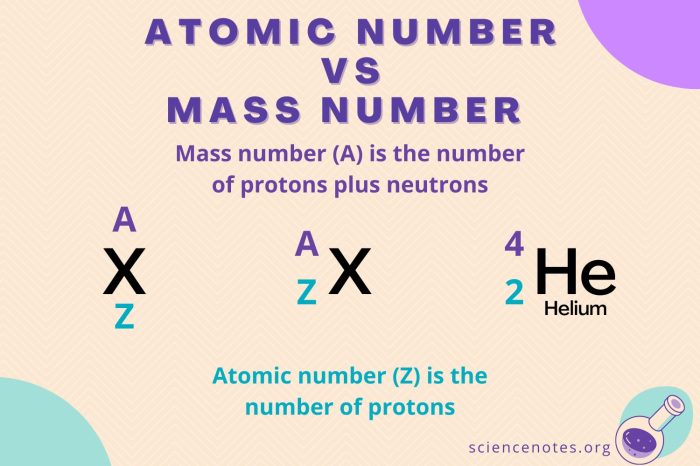
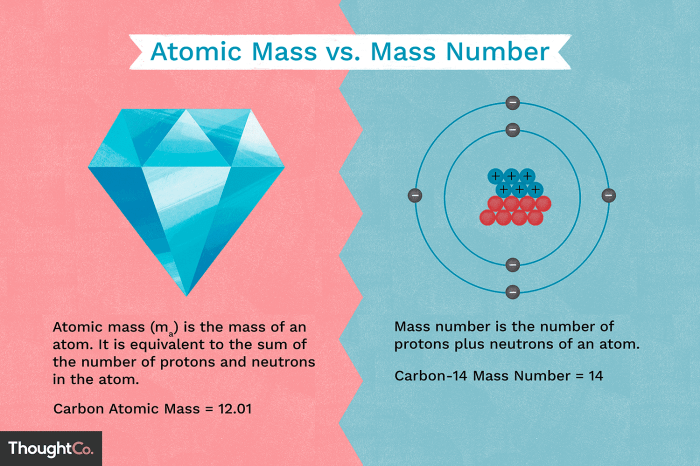
Mass number is the total number of protons and neutrons in the nucleus of an atom. It is represented by the symbol A.
For example, the mass number of carbon-12 is 12, indicating that a carbon-12 atom has 6 protons and 6 neutrons in its nucleus. The mass number of uranium-238 is 238, indicating that a uranium-238 atom has 92 protons and 146 neutrons in its nucleus.
The mass number of an atom is closely related to its atomic mass, which is the weighted average mass of all the isotopes of an element. The atomic mass of an element is typically expressed in atomic mass units (amu).
Worksheet Design
The worksheet should include practice problems related to atomic number and mass number. These problems could include:
- Multiple choice questions asking students to identify the atomic number or mass number of an element
- Fill-in-the-blank questions asking students to complete a table of atomic numbers and mass numbers
- Short answer questions asking students to explain the significance of atomic number and mass number
The worksheet should also include an answer key or answer guide for students to check their understanding.
Applications of Atomic Number and Mass Number
Atomic number and mass number are essential concepts in chemistry and other scientific fields. They are used in a variety of applications, including:
- Nuclear chemistry: Atomic number and mass number are used to identify and classify radioactive isotopes. This information is important for understanding the behavior of radioactive isotopes and for developing applications in medicine, energy production, and other fields.
- Medicine: Atomic number and mass number are used to develop diagnostic and therapeutic techniques. For example, the mass number of an element can be used to determine the density of a tissue, which can be helpful for diagnosing diseases such as cancer.
Question Bank
What is atomic number?
Atomic number refers to the number of protons within an atom’s nucleus, determining the element’s identity and position on the periodic table.
How is mass number calculated?
Mass number represents the total number of protons and neutrons found in an atom’s nucleus.
What is the relationship between atomic number and mass number?
While atomic number remains constant for a given element, mass number can vary due to the presence of different isotopes, which have varying numbers of neutrons.