Cu2+ and Zn2+ are examples of transition metal ions that play crucial roles in various chemical and biological processes. Their unique properties and applications make them essential components in many industrial and environmental contexts.
This article delves into the fascinating world of Cu2+ and Zn2+, exploring their properties, reactions, applications, and environmental implications. Join us on this journey to uncover the intriguing nature of these remarkable ions.
Definition of Cu2+ and Zn2+
Copper (II) ion (Cu2+) and zinc (II) ion (Zn2+) are positively charged metal ions with specific chemical properties and electron configurations.
The chemical symbol for copper (II) ion is Cu2+, indicating that it has lost two electrons from its neutral state. Similarly, the chemical symbol for zinc (II) ion is Zn2+, signifying the loss of two electrons.
Charges and Electron Configurations
The charges of Cu2+ and Zn2+ ions result from the loss of two electrons, giving them a +2 charge.
The electron configuration of Cu2+ is [Ar] 3d 9, indicating that it has lost two electrons from the 4s orbital and one electron from the 3d orbital.
Zn2+ has an electron configuration of [Ar] 3d 10, indicating the loss of two electrons from the 4s orbital.
Properties of Cu2+ and Zn2+
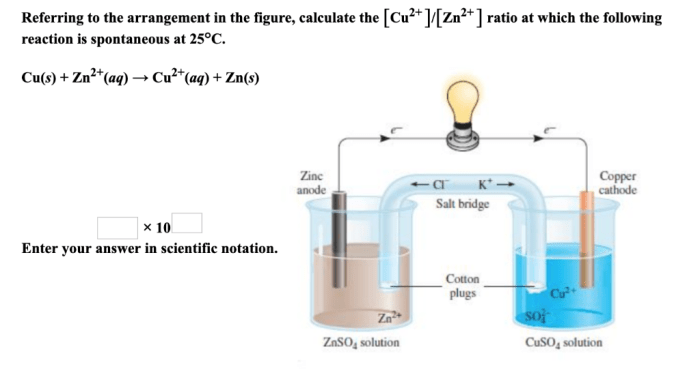
Cu2+ and Zn2+ are transition metal ions with distinct properties influenced by their electronic structures.
Color, Cu2+ and zn2+ are examples of
Cu2+ ions are typically blue or green due to the presence of unpaired d-electrons, which absorb light in the visible spectrum. In contrast, Zn2+ ions are colorless because they have no unpaired d-electrons and do not absorb visible light.
Solubility
Cu2+ ions are generally less soluble than Zn2+ ions in water. This difference in solubility is attributed to the stronger hydration energy of Cu2+ ions, which stabilizes them in aqueous solutions.
Magnetic Properties
Cu2+ ions are paramagnetic, meaning they are attracted to magnetic fields. This is because they have unpaired d-electrons that can interact with the magnetic field. Zn2+ ions, on the other hand, are diamagnetic, meaning they are not attracted to magnetic fields.
This is because they have no unpaired d-electrons.
Reactions of Cu2+ and Zn2+
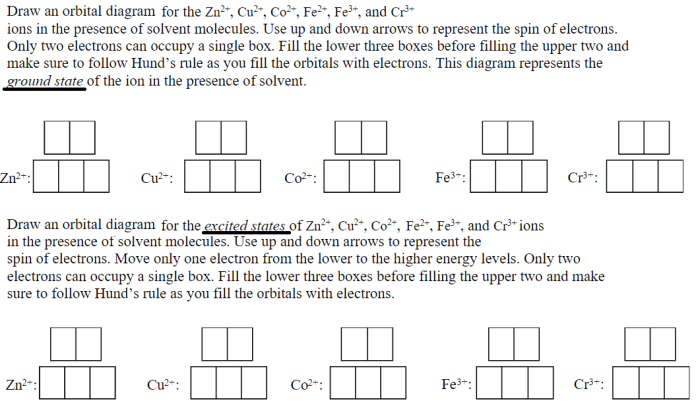
Copper(II) and zinc(II) ions can participate in redox reactions, where they act as oxidizing or reducing agents.
Redox Reactions Involving Cu2+
Copper(II) ions can undergo reduction to form copper(I) ions (Cu+). This reduction can be achieved by various reducing agents, such as iodide ions (I-), sulfite ions (SO32-), or metallic iron (Fe). For example, in the reaction between Cu2+ and I-, the Cu2+ ions are reduced to Cu+ ions, while the I- ions are oxidized to iodine (I2):
Cu2+ + 4I- → 2Cu+ + I2
In this reaction, Cu2+ acts as the oxidizing agent, accepting electrons from I- to form Cu+.
Redox Reactions Involving Zn2+
Zinc(II) ions can also undergo reduction to form zinc metal (Zn). This reduction can be achieved by stronger reducing agents, such as sodium metal (Na) or potassium metal (K). For example, in the reaction between Zn2+ and Na, the Zn2+ ions are reduced to Zn metal, while the Na atoms are oxidized to sodium ions (Na+):Zn2+ + 2Na → Zn + 2Na+In this reaction, Zn2+ acts as the oxidizing agent, accepting electrons from Na to form Zn metal.
Applications of Cu2+ and Zn2+
Copper(II) and zinc(II) ions have diverse applications in various industries and biological systems. They serve as catalysts, pigments, and essential nutrients.
Catalytic Applications
Cu2+ and Zn2+ are widely used as catalysts in chemical reactions. For instance, copper(II) sulfate is employed in the production of rayon, while zinc(II) chloride is utilized in the synthesis of organic compounds.
Cu2+ and Zn2+ are examples of transition metal ions. For more information about transition metals, check out unit 4 worksheet 3 chemistry . This resource provides a comprehensive overview of transition metals, including their properties and reactions.
Pigment Applications
Copper(II) and zinc(II) ions are incorporated into pigments to impart specific colors. Copper(II) ions are responsible for the blue-green color of malachite, while zinc(II) ions contribute to the white color of zinc oxide, commonly used in paints and sunscreen.
Biological Applications
Cu2+ and Zn2+ are essential for the proper functioning of biological systems. Copper(II) ions play a crucial role in oxygen transport and energy production, while zinc(II) ions are involved in numerous enzymatic reactions and immune function.
Environmental Impact of Cu2+ and Zn2+
Copper (Cu2+) and zinc (Zn2+) are essential elements for life, but at high concentrations, they can pose environmental hazards. These ions can enter aquatic ecosystems from various sources, such as industrial effluents, mining activities, and agricultural runoff.
Effects on Aquatic Ecosystems
In aquatic environments, Cu2+ and Zn2+ can accumulate in sediments and water, affecting the health and survival of aquatic organisms. Copper is particularly toxic to fish, even at low concentrations, and can cause damage to gills, liver, and kidneys. Zinc, while less toxic than copper, can still harm aquatic life by disrupting reproduction and growth.
Effects on Human Health
High levels of Cu2+ and Zn2+ in drinking water can pose health risks to humans. Copper can cause gastrointestinal distress, nausea, and vomiting. In severe cases, it can lead to liver and kidney damage. Zinc is less toxic than copper, but excessive intake can cause nausea, vomiting, and abdominal cramps.
Sources of Cu2+ and Zn2+ in the Environment
Industrial effluents
Copper and zinc are used in a wide range of industrial processes, including metalworking, electroplating, and battery manufacturing. Wastewater from these industries can contain high concentrations of these ions.
Mining activities
Mining operations can release copper and zinc into the environment through wastewater and runoff from tailings piles.
Agricultural runoff
Copper and zinc are used in fertilizers and pesticides, which can be washed away by rain and enter waterways.
Corrosion of copper pipes
Copper pipes used in plumbing can corrode over time, releasing copper ions into drinking water.
FAQ Compilation: Cu2+ And Zn2+ Are Examples Of
What is the difference between Cu2+ and Zn2+?
Cu2+ and Zn2+ are both transition metal ions with a +2 charge. However, they differ in their electronic configurations and properties. Cu2+ has an incomplete d-orbital, giving it paramagnetic properties and a characteristic blue color, while Zn2+ has a complete d-orbital, making it diamagnetic and colorless.
What are some common applications of Cu2+ and Zn2+?
Cu2+ is used as a catalyst in various industrial processes, such as the production of plastics and pharmaceuticals. It is also used as a pigment in paints and ceramics. Zn2+ is essential for biological processes, such as enzyme function and immune system regulation.
It is also used as a corrosion inhibitor and in the production of batteries.
What are the potential environmental hazards of Cu2+ and Zn2+?
Excessive levels of Cu2+ and Zn2+ in the environment can be toxic to aquatic organisms and can accumulate in the food chain. They can also contribute to water pollution and disrupt ecosystem balance.