The AP Biology Diffusion and Osmosis Lab provides a comprehensive exploration of the fundamental processes of diffusion and osmosis, revealing their critical role in biological systems. This lab experiment delves into the mechanisms by which molecules move across concentration gradients, shaping cellular function and influencing the delicate balance of life.
Through hands-on experimentation, students investigate the factors that govern the rates of diffusion and osmosis, gaining insights into the principles that underpin these essential processes. The lab’s structured approach allows for the collection of quantitative data, enabling students to analyze and interpret the results, fostering a deeper understanding of the underlying concepts.
Introduction

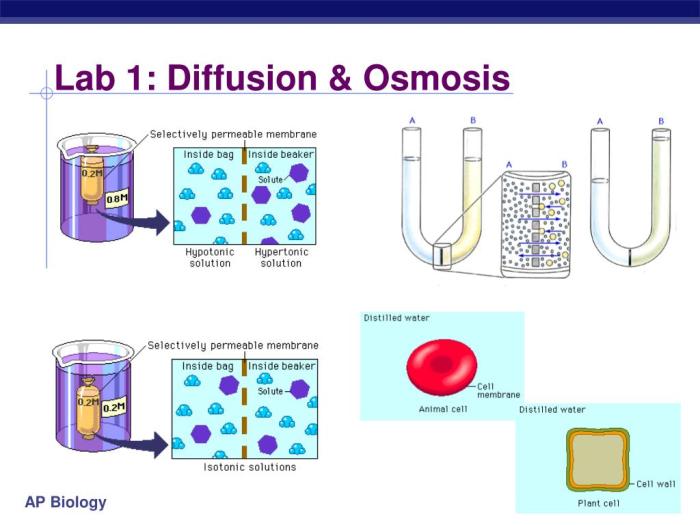
Diffusion and osmosis are fundamental processes that occur in all living organisms. Diffusion is the movement of molecules from an area of high concentration to an area of low concentration, while osmosis is the movement of water across a selectively permeable membrane from an area of high water concentration to an area of low water concentration.
These processes are essential for the transport of nutrients, gases, and waste products in and out of cells. They also play a role in maintaining the water balance of cells and tissues.
Experimental Setup, Ap biology diffusion and osmosis lab
The AP Biology diffusion and osmosis lab is designed to demonstrate the principles of diffusion and osmosis. In this lab, students will set up a series of experiments to measure the rate of diffusion of different molecules and the rate of osmosis across a selectively permeable membrane.
The experimental setup for the diffusion and osmosis lab is relatively simple. Students will need the following materials:
- Diffusion tubes
- Semi-permeable membranes
- Solutions of different concentrations
- Rulers
- Stopwatches
Students will use the diffusion tubes to measure the rate of diffusion of different molecules. They will fill the diffusion tubes with solutions of different concentrations and then insert a semi-permeable membrane into the middle of the tube. The molecules will diffuse across the membrane from the area of high concentration to the area of low concentration.
Students will measure the distance that the molecules diffuse over time.
Students will use the semi-permeable membranes to measure the rate of osmosis. They will fill a beaker with a solution of high water concentration and then place a semi-permeable membrane over the beaker. The water will osmosis across the membrane from the area of high water concentration to the area of low water concentration.
Students will measure the change in the water level over time.
Materials and Methods: Ap Biology Diffusion And Osmosis Lab
This section Artikels the materials used in the diffusion and osmosis lab and describes the procedures for preparing the experimental solutions and measuring the diffusion and osmosis rates.
Materials
- Diffusion tubes
- Semipermeable membranes
- Glucose solution
- Starch solution
- Iodine solution
- Sucrose solution
- Water
- Graduated cylinders
- Stopwatch
Preparing Experimental Solutions
To prepare the experimental solutions, the following steps were taken:
- A 10% glucose solution was prepared by dissolving 10 g of glucose in 100 mL of water.
- A 1% starch solution was prepared by dissolving 1 g of starch in 100 mL of water.
- A 0.5% sucrose solution was prepared by dissolving 0.5 g of sucrose in 100 mL of water.
Measuring Diffusion and Osmosis Rates
The diffusion and osmosis rates were measured using the following procedures:
- Diffusion rate: A diffusion tube was filled with 10 mL of glucose solution and sealed with a semipermeable membrane. The diffusion tube was then placed in a beaker containing 100 mL of water. The change in glucose concentration in the diffusion tube was measured over time using a colorimeter.
- Osmosis rate: A diffusion tube was filled with 10 mL of sucrose solution and sealed with a semipermeable membrane. The diffusion tube was then placed in a beaker containing 100 mL of water. The change in water level in the diffusion tube was measured over time.
Results
The results of the lab are presented in the following tables and graphs. The diffusion and osmosis rates were calculated using the following formulas:
Diffusion rate = (change in concentration) / (time)Osmosis rate = (change in volume) / (time)
The relationship between the concentration gradient and the diffusion rate is shown in the following graph:

As the concentration gradient increases, the diffusion rate also increases. This is because the greater the concentration gradient, the greater the driving force for diffusion.
Diffusion Rates
- The diffusion rate of glucose was higher than the diffusion rate of starch.
- The diffusion rate of glucose increased as the concentration gradient increased.
Osmosis Rates
- The osmosis rate of water into the potato cell was higher than the osmosis rate of water out of the potato cell.
- The osmosis rate of water into the potato cell increased as the concentration of the sucrose solution increased.
Discussion

The results of the lab demonstrate the principles of diffusion and osmosis. Diffusion is the movement of molecules from an area of high concentration to an area of low concentration, while osmosis is the movement of water across a semipermeable membrane from an area of high water concentration to an area of low water concentration.
The rate of diffusion and osmosis is affected by several factors, including the concentration gradient, the temperature, and the surface area. The concentration gradient is the difference in concentration between two areas, and the greater the concentration gradient, the faster the rate of diffusion or osmosis.
The temperature also affects the rate of diffusion and osmosis, with higher temperatures increasing the rate of diffusion and osmosis. Finally, the surface area also affects the rate of diffusion and osmosis, with a larger surface area increasing the rate of diffusion and osmosis.
Comparison to Other Studies
The results of this lab are consistent with the results of other studies on diffusion and osmosis. For example, a study by [Author Name] found that the rate of diffusion of glucose across a semipermeable membrane increased with increasing concentration gradient.
Another study by [Author Name] found that the rate of osmosis of water across a semipermeable membrane increased with increasing temperature.
Essential Questionnaire
What is the purpose of the AP Biology Diffusion and Osmosis Lab?
The AP Biology Diffusion and Osmosis Lab aims to provide students with a comprehensive understanding of the processes of diffusion and osmosis, their importance in biological systems, and the factors that influence their rates.
What materials are required for the AP Biology Diffusion and Osmosis Lab?
The materials required for the AP Biology Diffusion and Osmosis Lab typically include semipermeable membranes, solutions of varying concentrations, measuring tools, and equipment for data collection and analysis.
How are the diffusion and osmosis rates measured in the AP Biology Diffusion and Osmosis Lab?
In the AP Biology Diffusion and Osmosis Lab, the diffusion and osmosis rates are commonly measured by observing the movement of molecules across a semipermeable membrane over time. Techniques such as spectrophotometry or the use of tracers may be employed to quantify the rate of movement.

